Fuel Cells
Apr 1, 2009 12:00 PM, By Doug Irwin, CPBE AMD

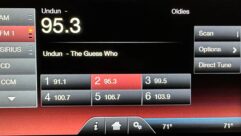
The components of a proton-exchange membrane fuel cell.
Click to enlarge
Undoubtedly while you carry out your day-to-day work around the radio station you use at least several rechargeable batteries. In a sense we are slaves to those batteries � because other technological considerations aside � if its battery is dead, it doesn’t matter how good and useful the technology of the device is. And to make matters worse, the energy we need to recharge those batteries (i.e. ac power) usually isn’t available where it is needed anyway, right? Otherwise we wouldn’t need the batteries in the first place.
Battery technology hasn’t kept up with the advancements in technology of the myriad of portable devices available now. Conventional wisdom is that the last important battery innovation was the introduction of the lithium-ion type, back in 1991. There have really only been incremental improvements since then; however, the late interest in electric vehicles has accelerated further developments. Very large versions of the lithium-ion battery type, for example, are expected to show up in hybrid vehicles this year.
The fact of the matter, though, is that all batteries run out of charge eventually. Wouldn’t it be great to have an alternative power source that was easily portable (unlike a generator), that worked day or night (unlike solar cells)?
Ever heard of fuel-cells?
Fuel-cells exist today, and have been used to generate power for decades. But for everyday applications, they aren’t quite ready for prime-time. Development is on-going, though, and it’s likely that we will be seeing fuel-cells come in to regular use in the not-too-distant future. In this article, we’ll take a look at their history, how they work, and the technological development of fuel-cells over the last 150 years. We’ll end by looking at the state of the technology in 2008.
Early work
The original idea behind the fuel cell is credited to the German Christian Friedrich Sch�nbein who published the idea in 1838. The following year, the Welsh engineer Sir William Grove demonstrated the first application of the idea.
In the 1930s, the British engineer Francis Bacon took up the idea, and developed it over the following two decades. In 1959 he successfully demonstrated a 5kW unit powerful enough to run an arc-welder. Pratt and Whitney licensed Bacon’s patents in the U.S. and they were subsequently used for the fuel-cells that flew in U.S. space missions in the 1960s. These fuel-cells generated electricity and the waste product of pure water.
Basically a fuel-cell is a device inside of which are three components: the anode, the cathode and electrically non-conductive separator. Both the anode and cathode are coated with a catalyst.
On the anode side, the hydrogen fuel is exposed to the catalyst, which encourages the disassociation of electrons from hydrogen atoms. The positive hydrogen ions will migrate through the separator; but, since the separator is electrically non-conductive, the electrons cannot move through it. This creates a potential difference (voltage) between the anode and cathode.
Fuel Cells
Apr 1, 2009 12:00 PM, By Doug Irwin, CPBE AMD

A direct-methanol fuel cell. The actual fuel cell stack is the layered element in the center.
When the anode and cathode are connected with a conductor, the electrons will move around the separator to the cathode, which is exposed to the oxidizer. The chemical reaction is completed; water is created as the byproduct. An electrical load can be connected between the anode and cathode, and therefore work can be done. This is what allows the fuel-cell to be a power source.
It’s important to note that this is an exothermic chemical reaction, which means that a substantial amount of heat is given off.
The potential between anode and cathode is on the order of 0.7V; and so just as in the case of batteries or photo-voltaic cells, the fuel-cells can be connected together in series and parallel, making a system with higher-voltage and greater current capability.
Various forms
The type of fuel-cell that uses pure hydrogen for the fuel is known as the proton-exchange membrane fuel-cell. (The proton-exchange membrane makes up the separator I referred to above.). There is another type of PEMFC that uses other hydrocarbons as fuel; the waste byproducts are then CO2 and water. (More on those below.) Operating temperature of a PEMFC is between 50 and 220 degrees Celsius.
One key component to a PEMFC is the catalyst, which is typically platinum or palladium. There is another type of fuel-cell, known as a solid-oxide fuel cell (or SOFC) that operates at a much higher internal temperature � 600 to 1000 degrees C. One advantage is that it does not need the catalyst on either the anode or cathode. The separator (as the name suggests) is ceramic. At these high temperatures, disassociated oxygen ions will move through the ceramic to oxidize the fuel on the anode side. Again, water is a byproduct. Connecting the cathode and anode together with a conductor allows electrons to flow; they can be used to do work just like any other electrical generator.
Hydrogen alternatives
As I mentioned above, it isn’t necessary to use pure hydrogen as fuel for a PEM fuel-cell.
Some manufacturers have worked to develop PEMFCs that use methanol as the fuel source. Methanol can be used to generate about 4.4kWh per liter of fuel. However, Lilliputian Systems of Wilmington, MA, has spent five years developing a matchbook-size SOFC that uses butane as its fuel. Butane, according to Lilliputian, is a better source of energy, since you can generate about 7.4kWh of power with one liter.
They make use of the intense heat to crack the butane molecules into hydrogen and carbon. The entire SOFC is enveloped in glass under a vacuum � much like a light bulb. Lilliputian expects to market the SOFC in 2009.
The Direct Methanol Fuel Cell type is an offshoot of the PEMFCs discussed herein. MTI Micro Fuel Cells of Albany, NY, has produced and demonstrated prototypes of its DMFC, mainly for use as battery replacements for electronic cameras and camcorders. Like Lilliputian, MFI expects their fuel-cell to reach the marketplace in 2009.
Toshiba has touted its DMFC fuel-cell technology for several years and has shown it at various technology showcases during that time. It showed a new cell-phone at CEATEC just this fall that uses a small DMFC to charge the lithium-ion battery that goes with the telephone. It has commenced mass production of the DMFC technology.
Panasonic (which recently officially changed its name from Matsushita) is producing a PEMFC for home use, with an output of 120Vac and a power capability of 1kW.
Panasonic has tested these units in Japanese households since 2005, and expects production in 2010. Some of its more interesting specifications: lifetime is 40,000 hours with 4,000 startup cycles (good enough for 10 years of service). It holds up to 200 liters of fuel (assuming you can find it) and it weighs 275 pounds � so don’t get too excited about buying one for use at remote broadcasts. Not yet anyway.
For the last three or four years, the introduction of the mass-production of small fuel-cells has been �right around the corner� so the current claims of all parties that 2009 is their target year need to be taken with the proverbial grain of salt. With the energy crisis of 2008, and the strong desire for more green technology from more and more consumers, and the ever-present desire to be freed from the tyranny of rechargeable batteries, it’s only a matter of time before small fuel-cells reach the market for real.
Irwin is transmission systems supervisor for Clear Channel NYC and chief engineer of WKTU, New York. Contact him at[email protected].